Manu Sharma, Ph.D.
Assistant Professor of Neuroscience
For more information: https://appel.weill.cornell.edu/labs/sharma-laboratory
Our Mission
My lab investigates pathogenesis of age-dependent neurodegenerative diseases, like Alzheimer's disease and Parkinson's disease, at the cellular-molecular level. To study these diseases, we combine biochemistry, cell biology, mouse genetic models and mouse behavior. Examples of some of these techniques are shown below:
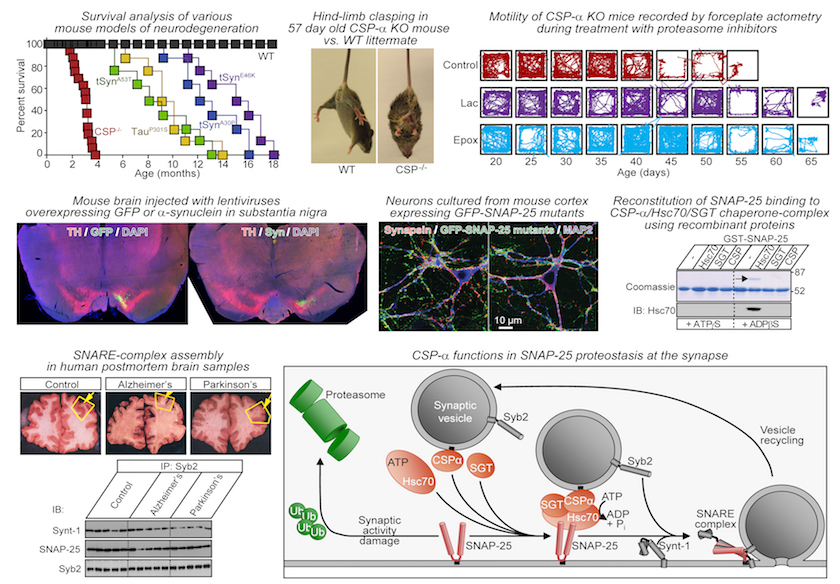
Short-term Goals
Neurodegenerative diseases are associated with varied genetic and environmental factors, yet age remains a universal risk factor. Age-associated failure of protein homeostasis, or “proteostasis”, causes disease-linked proteins to misfold into non-physiological oligomers and aggregates, producing a myriad of cellular dysfunctions. Long lifetime of a neuron enhances the need for proteostatic mechanisms, such as molecular chaperones that stabilize native protein folds and degradation pathways that eliminate misfolded proteins. My lab is interested in the mechanisms that mediate proteostasis in neurons and how their failure over time leads to neurodegeneration.
We are investigating this topic by:
a) Studying how proteostasis of Tau protein is regulated by two co-chaperones of Hsc70: CHIP (C-terminus of Hsc/Hsp70 interacting protein) and CSP-α (cysteine string protein-α).
b) Investigating how neurodegeneration is caused by recently found human mutations in CSP-α which cause neuronal ceroid lipofuscinosis, an adult-onset neurodegenerative disorder.
c) Comparing lysosomes from brains of normal mice and from neurodegenerative mouse models to identify changes that accompany the decline of neuronal proteostasis.
Long-term Goals
Our research will uncover proteostatic mechanisms in neurons which allow them to live and function for a lifetime, and how these mechanisms fail in aging neurons. Understanding neuronal proteostasis in health and disease may lead to treatment strategies for multiple neurodegenerative disorders, like Parkinson’s disease and Alzheimer’s disease, which share age-dependent proteostatic defects.
Recent Publications
- Burré J, Vivona S, Diao JJ, Sharma M, Brunger AT, Südhof TC, Properties of native brain α-Synuclein. Nature, 2013; 498(7453):E4-6.
- Pertsinidis A, Mukherjee K, Sharma M, Pang ZP, Park SR, Zhang Y, Brunger AT, Südhof TC, Chu S, Ultrahigh-resolution imaging reveals formation of neuronal SNARE/Munc18 complexes in situ. Proceedings of the National Academy of Sciences USA, 2013; 110(30):E2812-20.
- Burré J, Sharma M, Südhof TC. Systematic mutagenesis of α-synuclein reveals distinct sequence requirements for physiological and pathological activities. The Journal of Neuroscience, 2012; 32(43):15227-42.
- Sharma M*, Burré J, Südhof TC*. Proteasome inhibition alleviates SNARE-dependent neurodegeneration. Science Translational Medicine, 2012; 4(147):ra113. [* Corresponding authors]
- Sharma M*, Burré J, Bronk P, Zhang Y, Xu W, Südhof TC*. CSPα knockout causes neurodegeneration by impairing SNAP-25 function. EMBO Journal, 2011; 31(4):829-41. [* Corresponding authors]
- Etherton M, Földy C, Sharma M, Tabuchi K, Liu X, Shamloo M, Malenka RC, Südhof TC, Autism-linked neuroligin-3 R451C mutation differentially alters hippocampal and cortical synaptic function. Proceedings of the National Academy of Sciences USA, 2011; 108(33):13764-9.
- Etherton MR, Tabuchi K, Sharma M, Ko J, Südhof TC, An autism-associated point mutation in the neuroligin cytoplasmic tail selectively impairs AMPA receptor-mediated synaptic transmission in hippocampus EMBO Journal, 2011; 30(14):2908-19.
- Sharma M*, Burré J, Südhof TC*, CSPα Promotes SNARE-Complex Assembly by chaperoning SNAP-25 during synaptic activity. Nature Cell Biology, 2011; 13(1):30-9. [* Corresponding authors]
- Burré J#, Sharma M#, Tsetsenis T, Buchman V, Etherton MR, Südhof TC, α-Synuclein Promotes SNARE-Complex Assembly in vivo and in vitro. Science, 2010; 329(5999):1663-7. [# Equal contribution]

