M. Elizabeth Ross, M.D., Ph.D.
Nathan E. Cumming Professor of Neurology and Neuroscience
Chair, Neuroscience Graduate Program
Director, Center for Neurogenetics
Our Mission
The mission of the Ross laboratory is to study how genes direct the construction of cellular organization and connectivity of brain. Three major projects encompass: 1) neural tube formation, 2) the role of cell cycle regulation in patterning brain, and 3) cytoskeletal regulation in motile neurons required for migration and synaptogenesis. Our approach combines basic science and clinical genetic components.
Lab Members
Stanislav S. Kholmanskikh, PhD, Assistant Research Professor
Bozena S. Castaldo, MS, Lab Manager & Research Associate
Shenela Lakhani, MS, Director of Genetic Counseling & Instructor
Vanessa Aguiar-Pulido, PhD, Instructor of Neuroscience
Chengbing Wang, PhD, Senior Research Associate
Anna Schlusche, PhD, Postdoctoral Associate
Whitney Parker MD, PhD, Postdoctoral Associate
Jessica Kozikott, MHA, Administrator
Shawn Singh, BS, Research Specialist
Taylor Floyd, Graduate Student
Paul Wolujewicz, Graduate Student

Our Focus
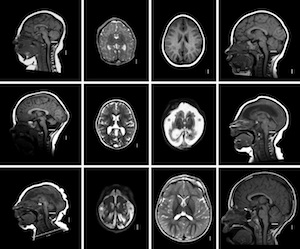
MR Images of microcephaly types
Microcephaly. The many genetic and environmental factors leading to microcephaly (small brain) together affect 2% of the population worldwide.Most of these disorders arise from failure of cell division to generate sufficient neurons and glia during embryonic and early postnatal development. Supported by multi-investigator NIH and Qatar Foundation grants our studies in neurogenesis were the first to recognize that the cell cycle protein, G1-phase active cyclin D2 (cD2) has an important role in brain formation. Loss of cD2 leads to cerebellar hypoplasia and microcephaly with selective interneuron deficits in both cerebellum and forebrain, due to the differential use of cD2 and cyclin D1 (cD1) in precursors of developing brain. These interneuron deficits lead to behavioral abnormalities and seizures due to inhibitory GABA deficits. Our studies show that cD2 expression is critical for intermediate progenitor proliferation in the mouse subventricular zone (SVZ) and that cD2 is the predominant cyclin expressed in the human fetal SVZ at 19 gestational weeks, suggesting particular importance of cD2 for human brain development. With collaborators, we have now identified gain of function mutations in cD2 in patients with a syndrome of megalencephaly (enlarged brain). Particularly in neurogenetic studies building at WCMC-Qatar, we are finding new gene associations causing structural brain disorders that we are studying in the lab.
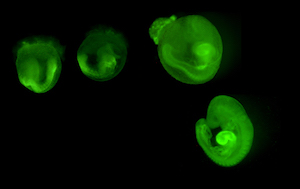
mouse embryos expressing myrVenus GFP
Spina bifida. Neural tube defects (NTDs), principally spina bifida and anencephaly, affect an estimated 140,000 or more live births annually worldwide. Using both animal models and human populations, we investigate the complex genetic and gene-environment interactions that predispose to NTDs. We showed that prenatal folic acid (FA) supplementation could prevent NTD in the Crooked tail (Cd) mouse strain in a manner that closely paralleled human clinical experience. We identified the Cd gene defect in the Lrp6 co-receptor required for Wnt signaling, an essential pathway in early brain development. We illuminated a previously unrecognized interaction between FA supplementation and Wnt signaling, demonstrating that both low and high FA levels attenuate the response to Wnt stimulus. This was the first demonstration that FA supplementation could have harmful effects on neural tube closure, depending on individual genetic background. We are now leading a multi-center clinical effort, supported by multi investigator grants from NIH, and support from the Qatar Foundation to discover genetic and epigenetic traits causing NTDs. Whole Genome Sequence analyses identify human polymorphisms, and ‘omics’ approaches seek epigenetic fingerprints and metabolic markers indicating increased NTD risk.

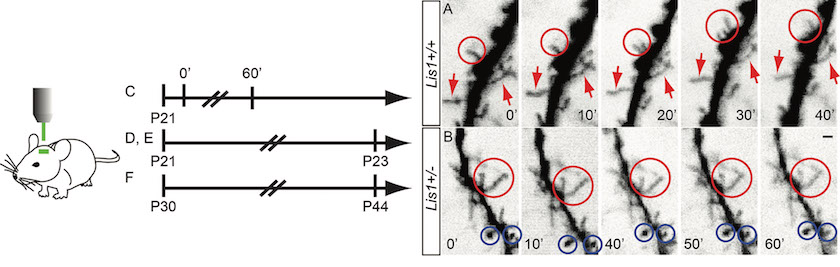
DTI images of mutant vs. wildtype mice
Epilepsy and Behavioral disorders relevant to Schizophrenia and the autism spectrum. We discovered the unexpected role of a gene associated with lissencephaly (smooth brain), Lis1, in signal transduction through small GTPases to the actin-based cytoskeleton of motile neurons. Lis1 participates in the dynein protein motor complex component of intracellular transport, and many consider Lis1 synonymous with dynein function. However, our studies indicate Lis1 is also required for signalling that modulates actin rich structures like growth cones and filopodia. We investigate the ability of Lis1 to regulate plasticity of neural networks and behavioral consequences relevant to schizophrenia, autism, epilepsy, and in our recent studies, dementia.
Future Goals
Our long term goal is to gain insights into the functional significance of human genetic variation and gene-environment interactions on the brain throughout the human life cycle.
To this end, we encompass human studies of population genetics, epigenetics and other “omics”, investigations in dissociated cell culture, tissues, and animal models including mouse and zebrafish to generate proof of principle insights and hypotheses.
Our ultimate goal is to bring these insights back to clinical use for disease risk assessment and individualization of disease prevention and treatment.
Achievements
- With collaborators, identified the doublecortin (DCX) gene and its mutations responsible for lissencephaly in boys and double cortex (subcortical band heterotopia) in girls.
- First to recognize the impact of Lis1 happloinsufficiency on the neuronal actin-based cytoskeleton and on Rho-family GTPase activities.
- First to show the importance of Lis1 expression in postmigrational neurons for synaptogenesis and plasticity
- First to identify the involvement of Wnt signaling molecule, low density lipoprotein receptor related protein 6 (Lrp6), in neural tube defects
- Demonstrated the ability of folic acid supplementation to either rescue NTDs or exacerbate embryopathy depending on the type of mutation in Lrp6 (gain of function vs loss of function)
- First to demonstrate that folic acid supplementation has a bi-modal effect on the robustness of canonical Wnt signaling.
- First to demonstrate the differential use of G1-active cyclin D1 and cyclin D2 (cD1 an cD2) in different neural progenitor populations of developing brain
- First to demonstrate that cD2 loss during development leads to disproportionately severe deficits in parvalbuninergic interneurons in forebrain and cerebellum
Selected Publications
- Vabres P, Sorlin A, Kholmanskikh SS, Demeer B, St-Onge J, Duffourd Y, Kuentz P, Courcet JB, Carmignac V, Garret P, Bessis D, Boute O, Bron A, Captier G, Carmi E, Devauchelle B, Genevieve D, Gondry-Jouet C, Guibaud L, Lafon A, Mathieu-Dramard M, Thevenon J, Dobyns WB, Bernard G, Polubothu S, Faravelli F, Kinsler VA, Thauvin C, Faivre L, Ross ME, Riviere JB. Postzygotic inactivating mutations of RHOA cause a mosaic neuroectodermal syndrome. (2019) Nat Genet. doi. 10.1038/s41588-019-0498-4
- Gupta I, Collier PG, Haase B, Mahfouz A, Joglekar A, Floyd T, Koopmans F, Barres B, Smit AB, Sloan SA, Luo W, Fedrigo O, Ross ME, Tilgner HU Single-cell isoform RNA sequencing characterizes isoforms in thousands of cerebellar cells. (2018) Nat Biotechnol. doi.10.1038/nbt.4259
- Sudarov A, Zhang XJ, Braunstein L, LoCastro E, Singh S, Taniguchi Y, Raj A, Shi SH, Moore H, Ross ME. Mature Hippocampal Neurons Require LIS1 for Synaptic Integrity: Implications for Cognition. (2018) Biol Psychiatry 83:518-529.
- Elsaid MF, Chalhoub N, Ben-Omran T, Kumar P, Kamel H, Ibrahim K, Mohamoud Y, Al-Dous E, Al-Azwani I, Malek JA, Suhre K, Ross ME†, Abdel Aleem A†. Mutation in non-coding RNA, RNU12 causes early-onset cerebellar ataxia. Ann Neurol. (2017) 81: 68-78. doi.10.1002/ana.24826
- Ross ME, Mason CE, Finnell RH. Genomic approaches to the assessment of human spina bifida risk. Birth Defects Res A Clin Mol Teratol. (2017) 109:120-128. doi. 10.1002/bdra.23592
- Marcucci F, Murcia-Belmonte V, Wang Q, Coca Y, Ferreiro-Galve S, Kuwajima T, Khalid S, Ross ME, Mason C, Herrera E. The Ciliary Margin Zone of the Mammalian Retina Generates Retinal Ganglion Cells. Cell reports (2016) 17:3153-3164.
- Petros TJ, Bultje RS, Ross ME, Fishell G, Anderson SA. Apical versus Basal Neurogenesis Directs Cortical Interneuron Subclass Fate. Cell reports (2015) 13:1090-1095
- Mirzaa GM*, Parry DA*, Fry AE*, Giamanco KA*, Schwartzentruber J, Vanstone M, Logan CV, Roberts N, Johnson CA, Singh S, Kholmanskikh SS, Adams C, Hodge RD, Hevner RF, Bonthron DT, Braun KP, Faivre L, Riviere JB, St-Onge J, Gripp KW, Mancini GM, Pang K, Sweeney E, van Esch H, Verbeek N, Wieczorek D, Steinraths M, Majewski J, Boycott KM, Pilz DT, Ross ME†, Dobyns WB, Sheridan EG†. De novo CCND2 mutations leading to stabilization of cyclin D2 cause megalencephaly-polymicrogyria-polydactyly-hydrocephalus syndrome. Nat Genet (2014); 46:510-515.
- Elsaid MF, Kamel H, Chalhoub N, Aziz NA, Ibrahim K, Ben-Omran T, George B, Al-Dous E, Mohamoud Y, Malek JA, Ross ME, Aleem AA. Whole genome sequencing identifies a novel occludin mutation in microcephaly with band-like calcification and polymicrogyria that extends the phenotypic spectrum. Am J Med Genet A (2014); 164A:1614-1617.
- Gilani AI, Chohan MO, Inan M, Schobel SA, Chaudhury NH, Paskewitz S, Chuhma N, Glickstein S, Merker RJ, Xu Q, Small SA, Anderson SA*, Ross ME*, Moore H*. Interneuron precursor transplants in adult hippocampus reverse psychosis-relevant features in a mouse model of hippocampal disinhibition. Proc Natl Acad Sci U S A (2014); 111:7450-7455.
- Sudarov A, Gooden F, Tseng D, Gan W-B, Ross ME. Lis1 controls dynamics of neuronal filopodia and spines to impact synaptogenesis and social behavior. EMBO Mol. Med. (2013); 5: 591-607.
- Gray JD, Kholmanskikh SS, Castaldo BS, Hansler A, Chung H, Brown AMC, Ross ME. Lrp6 Exerts Noncanonical Effects on Wnt Signaling During Neural Tube Closure. Hum. Mol. Genet. (2013); 22:4267-4281.
- RivièreJB, van BonWBM, HoischenA, KholmanskikhSS, O’RoakBJ, GilissenC, GijsenS, SullivanCT, ChristianSL, Abdul-RahmanOA, AtkinJF, ChassaingN, Drouin-GarraudV, FryAE, FrynsJ, GrippKW, KempersM, KleefstraT, ManciniGMS, NowaczykMJM, van Ravenswaaij-ArtsC, RoscioliT, RosenfeldJA, SiuVM, ShendureJ, VerloesA, VeltmanJA, BrunnerHG, Ross ME, Pilz DT, Dobyns WB. Exome sequencing implicates de novo mutations in the actin genes ACTB and ACTG1 in Baraitser-Winter syndrome. Nature Genet. (2012); 44:440-444.
Joint Appointments
Neurology
Center for Neurogenetics
https://neurogenetics.weill.cornell.edu/

