Karin Hochrainer, Ph.D.
Assistant Professor of Neuroscience
Lab Mission:
We aim to better understand how protein modifications impact neuronal viability in stroke and neurodegeneration, with the goal of harnessing this knowledge to improve the treatment of ischemic stroke and other neurodegenerative disorders.
Research Directions and Goals:
Ischemic Stroke:
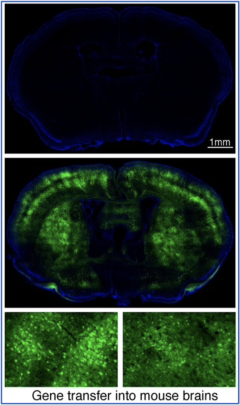 Ischemic stroke is a leading cause of death and disability worldwide, for which effective therapeutic interventions are lacking and thus direly needed. To achieve this, a better understanding of the molecular mechanisms of post-ischemic neuronal death is necessary. Ubiquitination, a post-translational protein modification with the small molecule ubiquitin, is essential for cell viability, particularly under stress conditions. Ischemic stroke poses a severe stress to affected brain cells, and we have found that ubiquitination is highly induced (Hochrainer et al, Stroke 2012, and JCBFM 2015). But how ubiquitination impacts post-ischemic protein function and in particular neuronal survival is unknown. By investigating this we hope to provide tools to modulate this response as a novel strategy for the treatment of ischemic stroke.
Ischemic stroke is a leading cause of death and disability worldwide, for which effective therapeutic interventions are lacking and thus direly needed. To achieve this, a better understanding of the molecular mechanisms of post-ischemic neuronal death is necessary. Ubiquitination, a post-translational protein modification with the small molecule ubiquitin, is essential for cell viability, particularly under stress conditions. Ischemic stroke poses a severe stress to affected brain cells, and we have found that ubiquitination is highly induced (Hochrainer et al, Stroke 2012, and JCBFM 2015). But how ubiquitination impacts post-ischemic protein function and in particular neuronal survival is unknown. By investigating this we hope to provide tools to modulate this response as a novel strategy for the treatment of ischemic stroke.
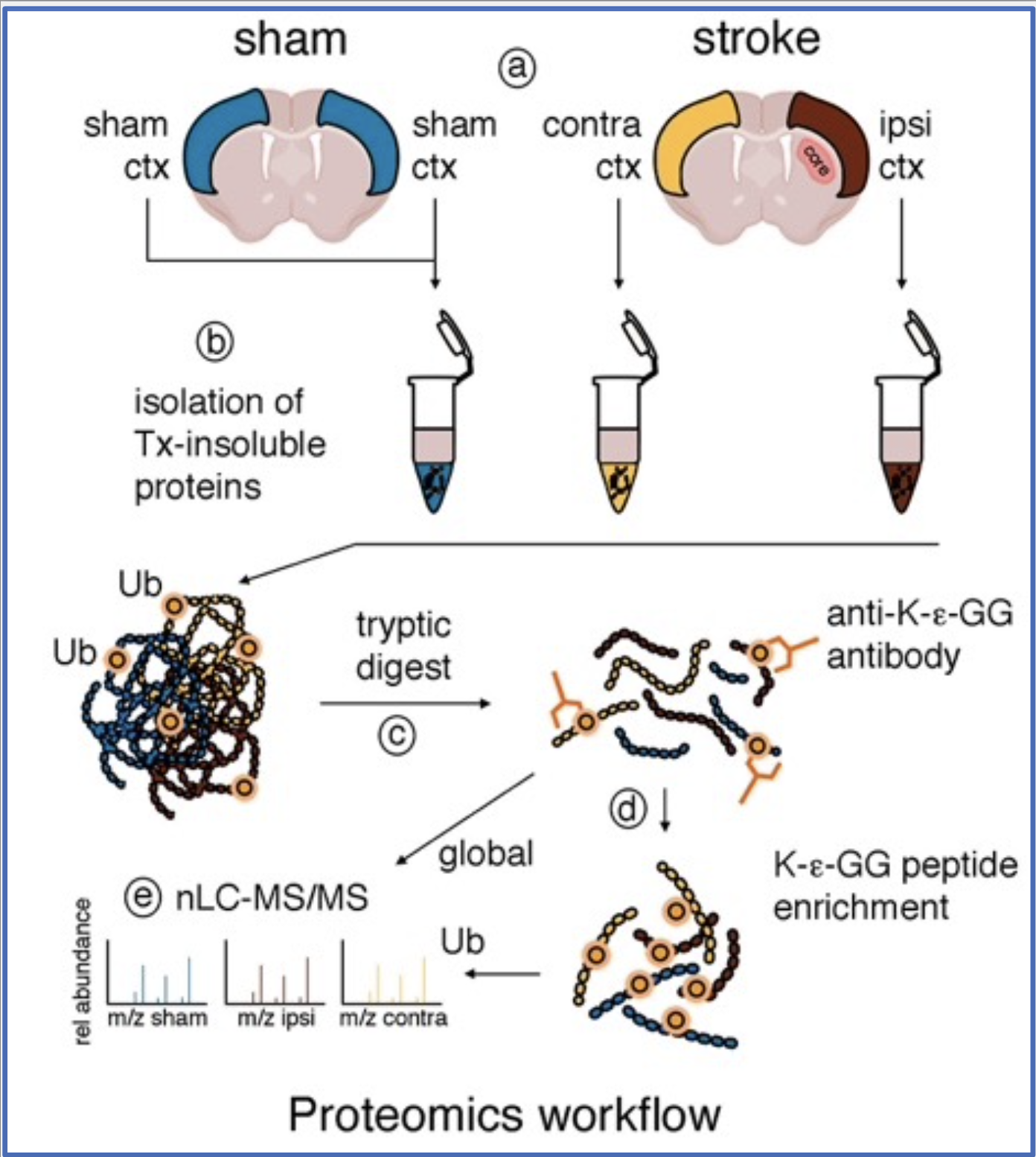
1. Post-ischemic ubiquitination targets:
To shed light on post-ischemic ubiquitination targets, we are performing proteomics on tissue derived from a mouse model of ischemic stroke. We are verifying targets and further characterizing them using cell culture, organotypic slice and animal stroke models, as well as gene transfer, gene knockdown and other molecular and biochemical wet lab techniques.
Our preliminary results suggest an enrichment of ubiquitinated proteins at the postsynaptic density (PSD), a highly specialized neuronal compartment whose function is essential for synaptic plasticity and neuronal survival. We are very excited about this finding as it implies a role for post-ischemic ubiquitination in neuronal communication, which may critically influence neuronal health after stroke. Much work lies ahead to dissect the impact ubiquitination has on ischemic targets.
2. Mechanisms leading to increased post-ischemic ubiquitination:
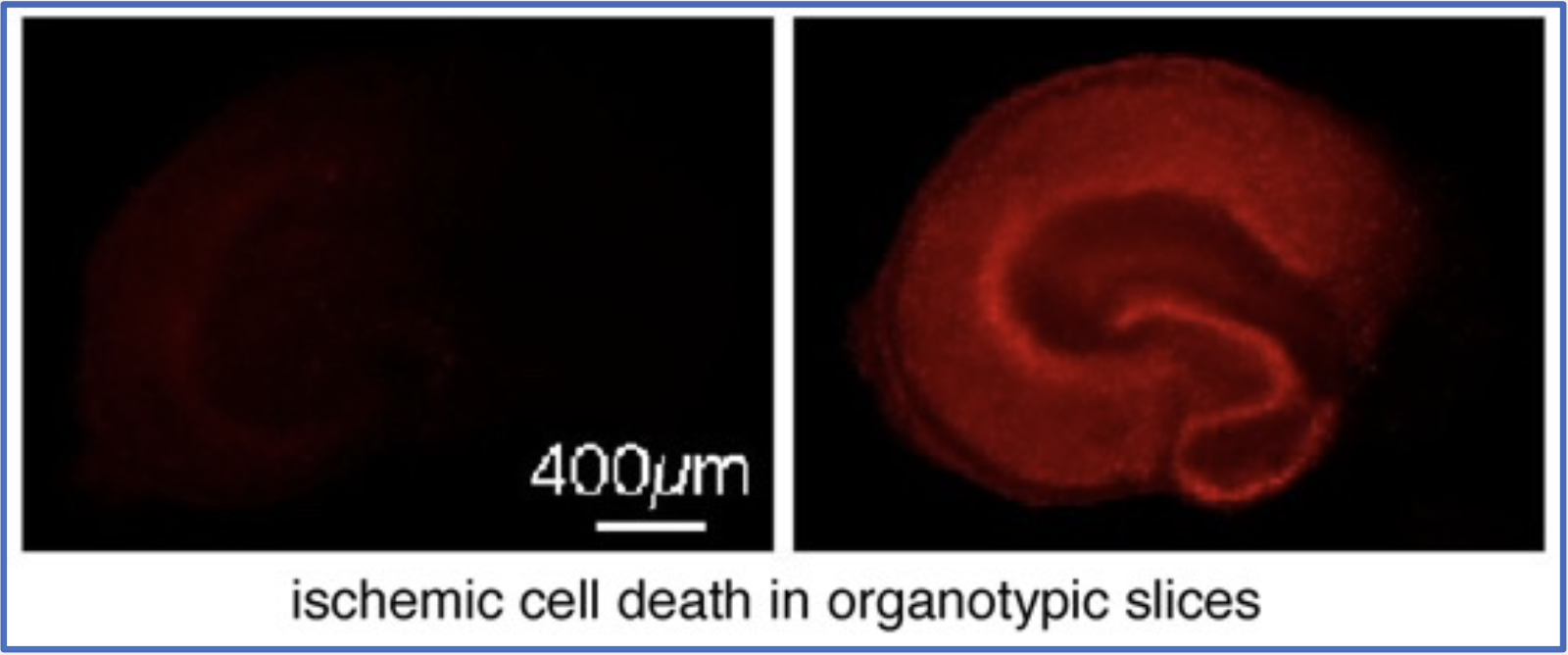
To be able to modify the ubiquitination response after ischemia we need to learn more about the involved mechanisms. Elevated ubiquitination in neurons often is caused by proteolysis failure, which is usually considered a sign of impending cell death. However, our recent work suggests that in case of ischemia the increase in ubiquitination is rather the result of a free radical-mediated reduction in intracellular deubiquitinase (DUB) activity (Kahles et al, CMLS 2021). This suggests a role of post-ischemic ubiquitination in protein regulation rather than proteostasis.
There are close to 100 DUBs in mammalian cells. Using siRNA screening approaches, we are now identifying those DUBs that impact post-ischemic ubiquitination and survival. The goal is to modify DUB activities and consequently neuronal death after stroke.
Tauopathy:
In collaboration with Dr Costantino Iadecola’s laboratory at WCM we investigate how the function of Tau, a protein involved in neurodegeneration, is altered early on in models of Tauopathy and Alzheimer’s disease.
3. Postsynaptic Tau and protein palmitoylation in Tauopathy:
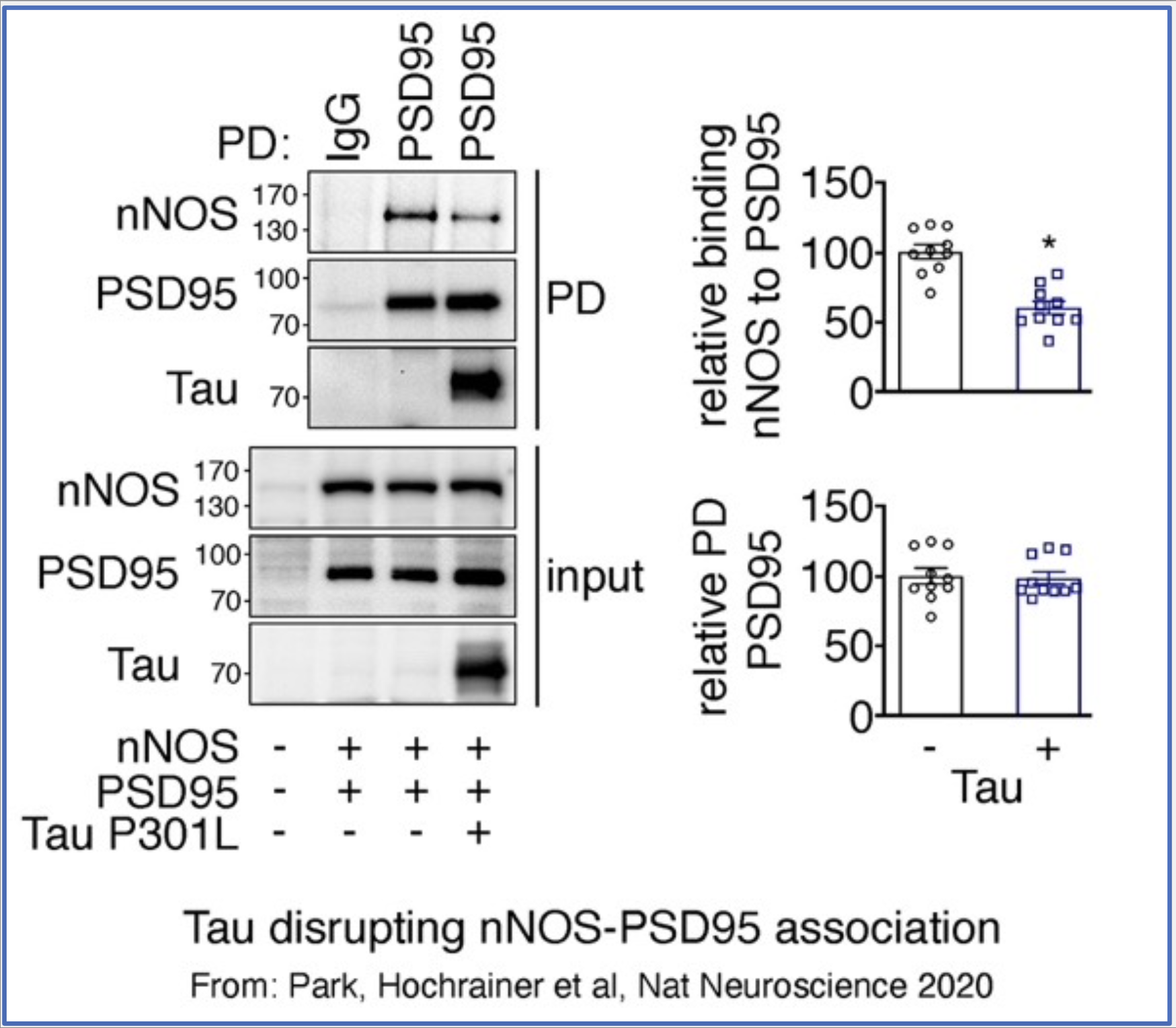
We unveiled a role of pathogenic Tau in disrupting PSD95-nNOS coupling at the postsynapse leading to decreased production of nitric oxide and neurovascular dysfunction (Park, Hochrainer et al, Nat Neuroscience 2020). These events occur before onset of neurodegeneration and provide a mechanism for neurovascular alterations that could be targeted for preclinical therapy.
Current efforts concentrate on uncovering the molecular determinants of Tau binding to PSD95, which seem to involve protein palmitoylation, a lipid modification of proteins. To study this, we are using cell culture as well as animal models of Tauopathy, combined with biochemistry, cell, and molecular biology techniques. The goal of this research is to find ways to interrupt this interaction and restore neurovascular function to prevent eventual cognitive decline.
Achievements:
*Discovered and partly characterized the protein family of small HERC HECT domain E3 ligases (PhD and PostDoc work)
*Established that the inflammatory mediator NF-kappaB is regulated by mono- and poly-ubiquitination, as well as acetylation and phosphorylation of the RelA subunit in a gene-specific manner (PostDoc and Early Career work).
*Discovered that ischemic stroke induces protein ubiquitination in the ischemic periphery dependent on reperfusion and independent of tissue damage.
*Established that deubiquitinase (DUB) inhibition by free radicals, and not proteasomal inhibition, is responsible for increases in post-ischemic ubiquitination.
*Revealed the insoluble accumulation of RNA-binding proteins commonly associated with neurodegeneration in acute ischemic stroke.
Lab Members:
Current:
*Emma Hambright, Research Technician I
*Luvna Dhawka, Research Technician I
*Gianfranco Racchumi, Laboratory Supervisor (in collaboration with Anrather Laboratory)
*Alicia Savage, Laboratory Manager (in collaboration with Iadecola Laboratory)
Past:
Research Technicians:
*Victoria Palfini (8/2018-6/2020, Research Technician I) – currently PhD student at Baylor College of Medicine
*Ismary Blanco (8/2016-6/2018, Research Technician I) - currently PhD student at Georgetown University
*Reunet Rodney-Sandy (8/2014-7/2016, Research Technician I) – obtained Medical Degree from St. George’s University School of Medicine
Undergraduate Trainees:
*Ashley Roth (6/2022-8/2022, Summer Intern from Northeastern University) – currently Research Assistant at Harvard Medical School
*Shanmukha Priya Srinivasan (1/2019-6/2019, Semester-long Intern from SASTRA University (India)) – currently PhD student at KU Leuven (Belgium)
*Shilpa Swaminathan (1/2018-6/2018, Semester-long Intern from SASTRA University (India)) - currently PhD student at the University of Queensland (Australia)
*Mohan Harihar Milaganur (1/2017-6/2017, Semester-long Intern from SASTRA University (India)) - currently PhD student at the University of Michigan
*Nefertari Duversaint (6/2016-8/2016, Summer Intern from CUNY)
*Jayashree Srinivasan (1/2016-6/2016, Semester-long Intern from SASTRA University (India)) - currently PhD student at University of Texas, Austin
*Vaishali Balachandran (1/2016-6/2016, Semester-long Intern from SASTRA University (India)) - currently Product Engineer at Berkeley Lights
*Habib Zahir (6/2015-8/2015, Summer Intern from Hunter College) - currently Resident Physician at Northwell Health
*Juhi Baskar (1/2015-6/2015, Semester-long Intern from SASTRA University (India)) – currently Software Engineer for Microsoft (India)
*Mehmet Kaplan (7/2013-9/2013, Summer Intern from Ege University (Turkey)) - currently PostDoc at the Medical University of Innsbruck (Austria)
*Victoria Olaseun (5/2013-8/2013, Summer Intern from Lehman College) – currently Research Technician at Weill Cornell Medicine
Highschool Trainees:
*Sittha Cheerasarn (7/2022-9/2022, Summer Intern Bronx High School of Science)
*Diego Santisteban Vargas (7/2019-7/2019, Summer Intern from Costa Rica)
Selected Publications:
- Hochrainer K, Yang W. Stroke Proteomics: From Discovery to Diagnostic and Therapeutic Applications. Circ Res. 2022; 130(8): 1145-1166.
- Kahles T, Poon C, Qian L, Palfini V, Srinivasan SP, Swaminathan S, Blanco I, Rodney-Sandy R, Iadecola C, Zhou P, Hochrainer K. Elevated post-ischemic ubiquitination results from suppression of deubiquitinase activity and not proteasome inhibition. CMLS. 2021; 78(5): 2169-83.
- Park L, Hochrainer K, Hattori Y, Ahn SJ, Anfray A, Wang G, Uekawa K, Seo J, Palfini V, Blanco I, Acosta D, Eliezer D, Zhou P, Anrather J, Iadecola C. Tau induces PSD95-nNOS uncoupling and neurovascular dysfunction independent of neurodegeneration. Nat. Neurosci 2020; 23(9): 1079-89.
- Faraco G, Hochrainer K, Segarra SG, Schaeffer S, Santisteban Calvo M, Menon A, Jiang H, Holtzman DM, Anrather J, Iadecola C. Dietary salt promotes cognitive impairment through tau phosphorylation. Nature 2019; 574(7780): 686-690.
- Kahl A, Blanco I, Jackman K, Baskar J, Milaganur Mohan H, Rodney-Sandy R, Zhang S, Iadecola C, Hochrainer K. Cerebral ischemia induces the aggregation of proteins linked to neurodegenerative diseases. Sci Rep. 2018; 8(1): 2701.
- Hochrainer K. Protein modifications with ubiquitin as response to cerebral ischemia-reperfusion injury. Transl Stroke Res. 2018; 9(2): 157-173.
- *Hochrainer K, Pejanovic N, Olaseun VA, Zhang S, Iadecola C, Anrather J. The ubiquitin ligase HERC3 attenuates NF-κB-dependent transcription independently of its enzymatic activity by delivering the RelA subunit for degradation. Nucleic Acids Res. 2015; 43(20): 9889-904. (*corresponding author).
- *Hochrainer K, Jackman K, Benakis C, Anrather J, *Iadecola C. SUMO2/3 is associated with ubiquitinated protein aggregates in the mouse neocortex after middle cerebral artery occlusion. JCBFM 2015; 35(1): 1-5. (*co-corresponding authors).
- Hochrainer K, Jackman K, Anrather J, Iadecola C. Reperfusion rather than ischemia drives the formation of ubiquitin aggregates in the mouse neocortex after middle cerebral artery occlusion. Stroke 2012; 43(8): 2229-2235.
- Hochrainer K, Racchumi G, Zhang S, Iadecola C, Anrather J: Monoubiquitination of nuclear RelA negatively regulates NF-κB activity independent of proteolysis. CMLS. 2012; 69(12): 2057–2073.
For a complete publication list please visit:
https://www.ncbi.nlm.nih.gov/myncbi/karin.hochrainer.1/bibliography/public/
Research Support:
National Institute of Neurological Disorders and Stroke
American Heart Association
Kenny Foundation
Sackler Brain and Spine Institute
Feil Family Foundation
Presidents Council of Cornell Women
Harriman Foundation
Collaborators:
Costantino Iadecola (BMRI)
Josef Anrather (BMRI)
Ping Zhou (BMRI)
Ulrike Resch (Medical University of Vienna, Vienna, Austria)
Agnidipta Ghosh (Albert Einstein School of Medicine, NY, USA)

