Teresa A. Milner, Ph.D.
Professor of Neuroscience
Our Mission
The Teresa A. Milner laboratory aims to understand estrogen influences on the brain function over the life-cycle.

Lab Members
Tracey Van Kempen (graduate student)
Jose Da Silva Marques Lopes (Post-doc)
Goals
To test the hypothesis that chronic stress leads to adaptive changes in the hippocampal opioid system of females to promote CA3 long-term potentiation and other plastic processes that support drug-related associative learning. This study uses a combination of immuno-electron microscopic and physiological approaches in rats.
To test the central hypothesis that changes in postsynaptic NMDA receptors and associated signaling pathways with estrogen receptor-beta neurons in the paraventricular hypothalamic nucleus during menopause predispose these neurons to increase excitability in response to hypertensive challenges. This study uses a combination of immuno-electron microscopic, molecular and physiological approaches in transgenic mice.
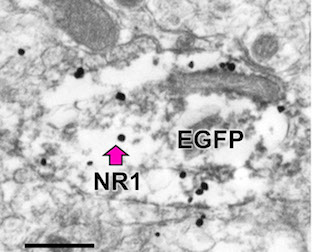
Ultrastructural analysis of the mouse hypothalamic paraventricular nucleus shows that NR1-silver-intensified immunogold (black dots) density is decreased in estrogen receptor β-enhanced green fluorescent protein (EGFP)-containing dendrites in young females, and increased in males and aged females.
Throughout the life cycle, estrogens and other gonadal steroids can influence many brain functions. In addition to regulating reproductive functions and homeostasis, estrogens can affect cognitive and emotional processes and autonomic functions. My research focuses on delineating the mechanisms by which estrogens influence cognition, particularly that related to drug abuse, and cardiovascular functions during menopause.
Women are more susceptible to several aspects of drug addiction than men, including relapse following stressful events. The hippocampus is a brain region that is critically involved in learning relevant to drug abuse. Moreover, estrogens and opioid peptides by binding to select receptors can modulate learning processes in the hippocampus. Over the years, my lab has localized estrogen and opioid receptors in rodents using electron microscopic immunocytochemical methods to help elucidate the mechanisms by which estrogens and opioids interact to impact learning relevant to drug abuse. In particular, we have found that estrogen receptors (ERs) are not only found in nuclei where they can influence genomic events but also are located in synapses where they can influence rapid communication between nerve cells. We also have demonstrated that the mu- and delta-opioid receptors (MOR and DORs, respectively) are present on select subtypes of hippocampal neurons where they can influence the balance of excitation and inhibition. My most recent studies indicate that estrogens regulate endogenous hippocampal opioid peptides and MORs and DORs in a manner that could promote learning processes relevant to drug abuse and relapse after chronic stress.
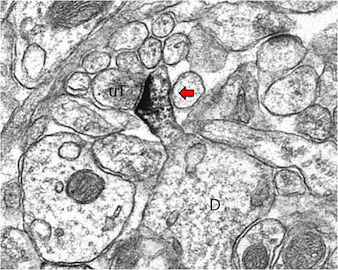
Estrogen receptor beta (red arrow) is located in the spine of a dendrite that is contacted by an unlabeled terminal (uT) in the rodent hippocampus. The localization of estrogen receptors in synaptic compartments supports local actions of estrogens. Milner et al. J Comp Neurol 2005
After menopause, hypertension and stress reactivity increases in women. My lab investigates the central mechanisms by which estrogens regulate blood pressure in rodents to help understand the development of hypertension and stress reactivity in menopause. We have shown that receptors for estrogens and other ovarian steroids have a regionally selective location relative to cardiovascular brain circuits. Using combined neuroanatomical and physiological approaches, we have shown that estrogens, primarily via ER beta, regulate angiotensin-signaling in the rostral ventrolateral medulla, a brain region crucial for the regulation of blood pressure. My most recent research utilizes a new “accelerated ovarian failure” model of menopause to study the mechanisms by which changes in estrogen levels during menopause influences cells the hypothalamic paraventricular nucleus, a brain region critical for integrating and coordinating neurohumoral responses involved in blood pressure regulation. These studies have contributed importantly to understanding changes in the brain during menopause that contribute to the increase susceptibility to hypertension.
Achievements
Using immuno-electron microscopy we discovered that estrogen and progestin receptors are found at extranuclear sites in dendritic spines, terminals and glia where they can affect local cell signaling.
Using a multidisciplinary approach our studies have shown that the opioid system in the female hippocampus is primed for enhance associative learning processes after chronic stress.
We have characterized a novel mouse model of menopause and are currently using it to elucidate mechanisms of hypertension maintenance in the hypothalamus.
Recent Publications
- Burstein, S.R., Williams, T.J., Lane, D.A., Knudsen, M.G., Pickel, V.M., McEwen, B.S., Waters, E.M., and Milner, T.A.: The influences of reproductive status and acute stress on the levels of phosphorylated delta opioid receptor immunoreactivity in rat hippocampus. Brain Res. 1518: 71-81 (2013) PMID: 23583481; PMCID: PMC3764923.
- Eagleson, K.L., Milner, T.A., Xie, Z., and Levitt, P.: Synaptic and extrasynaptic location of the receptor tyrosine kinase Met during postnatal development in the mouse neocortex and hippocampus. J. Comp. Neurol. 521: 3241-3259 (2013) PMID: 23787772; PMCID: PMC – in process.
- Milner, T.A., Burstein, S.R., Marrone, G.F., Khalid, S., Gonzalez, A.D., Williams, T.J., Schierberl, K.C., Torres-Reveron, A., Gonzales, K.L., McEwen, B.S., and Waters, E.M.: Stress differentially alters mu opioid receptor density and trafficking in parvalbumin-containing interneurons in the female and male rat hippocampus. Synapse 67(11): 757-752 (2013); PMID: 23720407; PMCID: PMC3778032
- Mitterling, K.L., Spencer, J.L., Dziedzic, N., Shenoy, S., McCarthy, K., Waters, E.M., McEwen, B.S., and Milner, T.A.: Cellular and subcellular localization of estrogen and progestin receptor immunoreactivities in the mouse hippocampus. J. Comp. Neurol. 518: 2729-2743 (2010) PMID: 20506473; PMCID: PMC2879091
- Waters, E.M., Yildirim, M., Janssen, W.G.M., Lou, W.Y.W., McEwen, B.S., Morrison, J.H., Milner, T.A.: Estrogen and aging affect the synaptic distribution of estrogen receptor beta immunoreactivity in the CA1region of female rat hippocampus. Brain Res. Special Issue “Window of Opportunity” 1379: 86 – 97 (2011) PMID: 20875808; PMCID: PMC3046233
- Van Kempen, T.A., Milner, T.A., and Waters, E.M.: Accelerated ovarian failure: a novel, chemically-induced animal model of menopause Brain Res. Special Issue “Window of Opportunity” 1379: 176 – 187 (2011) PMID: 21211517; PMCID: PMC3078694
- Williams, T.J., Torres-Reveron, A., Chapleau, J.D. and Milner, T.A.: Hormonal regulation of delta opioid receptor immunoreactivity in interneurons and pyramidal cells in the rat hippocampus. Neurobiol. Learning & Memory 95 (2): 206-220 (2011) PMID: 21224009; PMCID: PMC3045654
- Spencer, J.L., Waters, E.M., Bath, K.G., Chao, M.V., McEwen, B.S., and Milner, T.A.: Distribution of phosphorylated TrkB receptor in the mouse hippocampal formation depends on sex and estrous cycle stage. J. Neurosci. 31: 6780-6790 (2011) PMID: 21543608; PMCID: PMC3108038
- Gonzalez, A.D., Wang, G., Waters, E.M., Gonzales, K.L., Speth, R.C., Van Kempen, T.A., Marques-Lopes, J., Young, C.N., Butler, S.D., Davisson, R.L., Iadecola, C., Pickel, V.M., Pierce, J.P., and Milner, T.A.: Distribution of angiotensin type 1a receptor containing cells in the brains of bacterial artificial chromosome transgenic mice. Neuroscience 226: 489-509 (2012) PMID: 22922351; PMCID: PMC3505886.
Marques-Lopes, J., Van Kempen, T.A., Waters, E.M., Pickel, V.M., Iadecola, C., and Milner, T.A.: Slow-pressor angiotensin II hypertension and concomitant dendritic NMDA receptor trafficking in estrogen receptor beta-containing neurons of the mouse hypothalamic paraventricular nucleus are sex and age dependent. J. Comp. Neurol. (2014) in press
Joint Appointments
Adjunct Professor, Laboratory of Neuroendocrinology, The Rockefeller University
Collaborators
Jochen Buck
Robin Davisson
Michael Glass
Barbara Hempstead
Costantino Iadecola
Diane Lane
Francis Lee
Lonny Levin
Virginia Pickel
Anjali Rajadhyaksha
Gang Wang

